NEW STUDY ANNOUNCEMENT
Levagen+ for Diabetic Peripheral Neuropathy Study
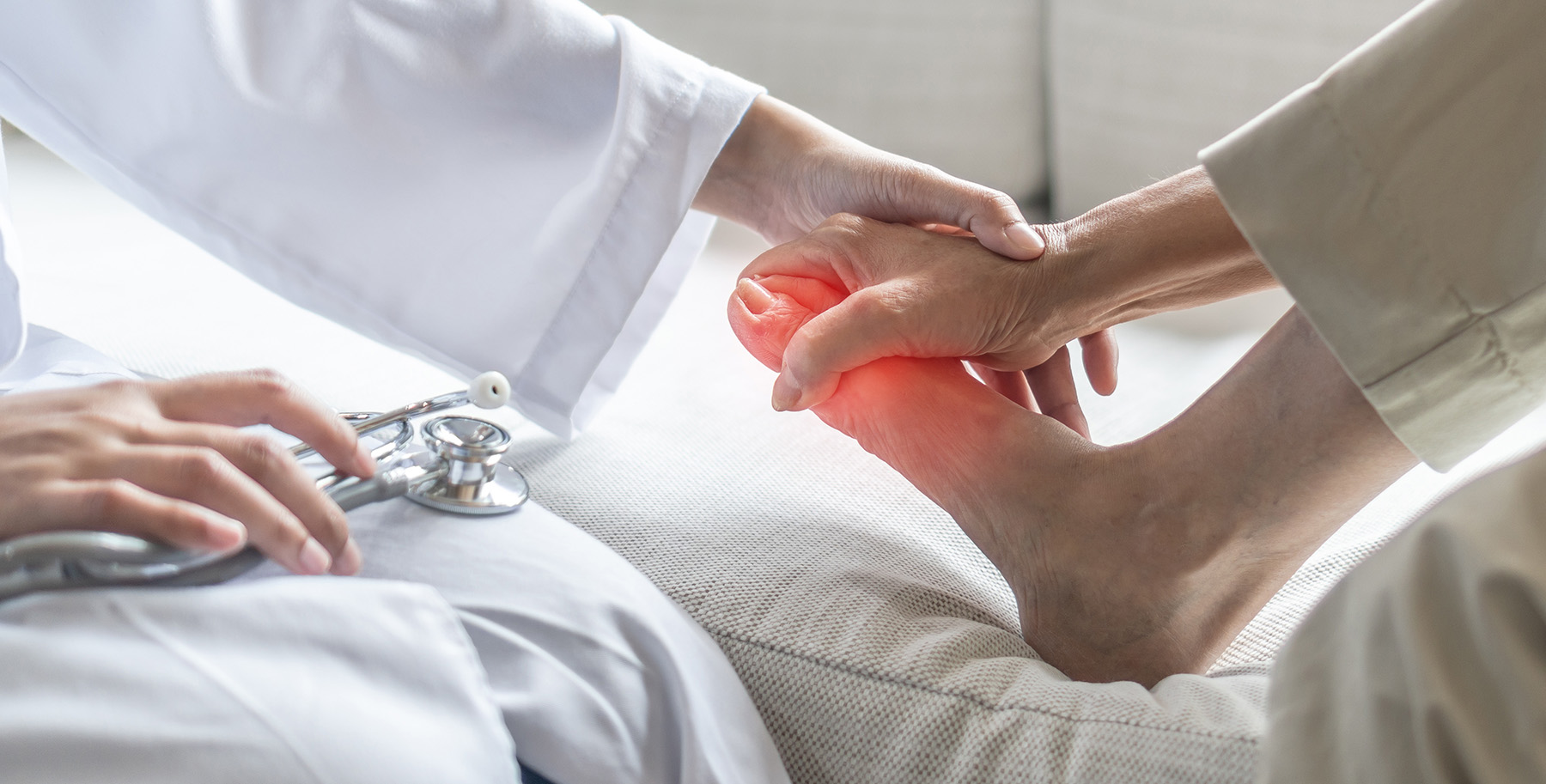
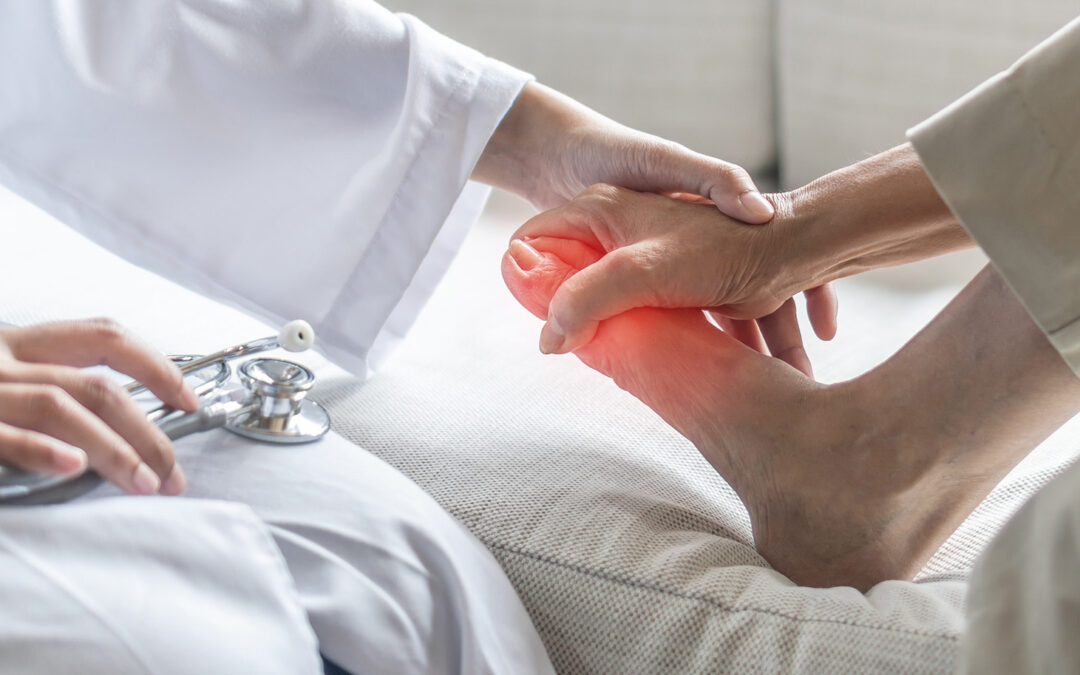
Are you a diabetic aged between 18-75 and experiencing symptoms of peripheral neuropathy such as tingling and burning pains in the hands and feet, pins and needles, numbness, muscle weakness, cramping, and poor balance?
Thank you for your interest in our study which is investigating the efficacy of a supplement containing palmitoylethanolamide (PEA) in reducing the symptoms of peripheral neuropathy in diabetics.
Please note: This page is a summary of the study outline and participant requirements. A full Participant Information Sheet with detailed information will be provided to you before you are asked to give your consent to participate.
Why is this study being conducted?
Diabetic peripheral neuropathy affects up to half of all people experiencing either type 1 or type 2 diabetes mellitus. It can produce symptoms such as tingling and burning pains in the hands and feet, pins and needles, numbness, muscle weakness, cramping, and poor balance. It can also negatively affect sleep, mood and overall quality of life.
Current treatment options for diabetic peripheral neuropathy include prescription medications, however they may be associated with side effects, and their long-term effectiveness and safety is unclear. Therefore, it is important to investigate alternative treatment options for this condition.
How long is the study, and when does it start?
Your involvement in this study will be 12 weeks, during which time you will be required to take the study product (1 capsule) twice daily. Most of the study assessments can be completed from home, however you will be asked to visit an ACL pathology centre on three occasions for to provide a fasted blood sample.
We are recruiting on a rolling basis which means that your individual start date is flexible.
For your convenience, you can attend any ACL pathology collection centre for your blood test.
Please see below for more information. You can also register your interest using the form at the bottom of this page.
About the product you’ll help investigate…
This study is designed to examine the effects of a supplement called Levagen+ in people with diabetic peripheral neuropathy. Levagen+ is a natural supplement containing PEA, which is a fatty acid derivative with anti-inflammatory and pain reducing properties. Previous studies have shown promising results for PEA supplementation in people suffering from painful conditions such as diabetic peripheral neuropathy.
Levagen+ is vegan friendly.
The product will be supplied in capsule form, and participants will be asked to take one capsule twice daily with food, for 12 weeks.
As this study is placebo-controlled, you may or may not be allocated the active product. The product is allocated randomly on a chance basis, and you will not know which product group you have been allocated to. This is to ensure that no unintentional bias can impact the results.
In this study, there is 1 active group and 1 placebo group, so you will have a 50% chance of receiving the active product.
Placebos are designed to look like the real product being studied except they do not contain the active ingredient. Using a placebo helps us to be confident that any effects we measure in the active group really are due to the active ingredient and not due to the body’s own reaction to a perceived treatment
What is required of participants?
Your involvement in the study will be for 12 weeks. Most of the study requirements can be completed online at home; however, you will be required to attend your local ACL pathology centre for a blood test 3 times during the study.
During the study, you will be asked to:
- Take the study product (in capsule form) twice daily with food for 12 weeks.
- Attend an ACL pathology centre 3 times for a fasted blood test.
- Complete a series of short online questionnaires at 5 points throughout the study.
The information we collect during the study will enable the investigators to assess the product’s tolerability and efficacy in improving the symptoms associated with diabetic peripheral neuropathy.
To make the timings of these visits as convenient as possible for you, we are recruiting on a rolling basis. This means that your individual start date is flexible. And you can attend any ACL pathology centre for the blood test.
Please note: This page is a summary of the study outline and participant requirements. A full Participant Information Sheet with detailed information will be provided to you before you are asked to give your consent to participate.
COMPENSATION
Upon completion of the study requirements, you will be eligible to be paid for your time and travel expenses. The payment will be made by gift voucher or via direct deposit to your nominated bank account (your choice) once all study requirements have been met.
Pro rata payments are also available in the case of early withdrawal.
If you are interested in participating, we’d love to hear from you. Please register your interest by answering the questionnaire and completing the form on this page…we’ll then get in touch to screen you for eligibility.
Check if you can register to take part…
- Adults 18-75 years of age
- Experiencing symptoms of diabetic peripheral neuropathy such as tingling and burning pains in the hands and feet, pins and needles, numbness, muscle weakness, cramping, and poor balance.
- Using prescribed medications or insulin for the management of diabetes (Type 1 or Type 2)
- Generally healthy
- Able to provide informed consent
- Able to commit to completing the study requirements
Please register your interest by answering the questionnaire and completing the form on this page… we’ll then get in touch to screen you for suitability.
Please note: This page is a summary of the study outline and participant requirements. A full Participant Information Sheet with more detailed information will be provided to you before you are asked to give your consent to participate.
Ethics approval and privacy
This RDC Clinical study has been approved by the Bellberry Human Research Ethics Committee under Approval Number: 2024-12-2103. The study is also registered on the clinicaltrials.gov under Registration Number: NCT06827327
Trained trial coordinators will supervise your progress throughout the study. Your results will be made available to you at the conclusion of the study. Any of your personal information collected during the study will be kept in the strictest confidence.